Abstract
Introduction
Diffuse large B-cell lymphoma of the ocular adnexa (OA-DLBCL) is extremely rare and historically was associated with poor prognosis and higher risk of central nervous system (CNS) relapse. In the rituximab era its prognosis may be more favorable, particularly in individuals with limited-stage disease. However, published series of patients with extranodal limited-stage DLBCL, including a study from our group (Bobillo et al. Blood 2021), rarely included OA-DLBCL. Studies specifically looking at OA-DLBCL reported diverse treatment approaches, including some that are no longer current, such as chemotherapy without rituximab or radiation alone. Thus, the optimal management of limited-stage OA-DLBCL remains poorly defined. To address this knowledge gap, we reviewed treatments and outcomes of OA-DLBCL patients treated at Memorial Sloan Kettering Cancer Center (MKSCC).
Methods
We retrospectively reviewed electronic medical records of consecutive patients with DLBCL managed at MSKCC who had ocular adnexal involvement and stage IE or IIE disease, and complete clinical information. Patients with stage III-IV disease, or evidence of intraocular or CNS involvement were excluded.
Results
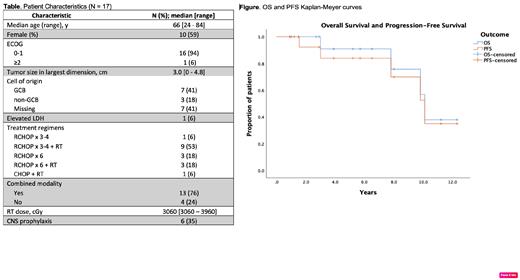
Between 2000 and 2020 we identified 17 patients with limited-stage OA-DLBCL. The median age at diagnosis was 66 years [range: 24-84], all had ECOG PS 0-1, 94% had IPI 0-1 and 94% CNS-IPI 0-1. Primary sites of ocular adnexal involvement included: extraconal orbit (9), intraconal orbit (2), lacrimal gland (4), and eyelid (2). Median greatest diameter of lesions was 3.0 cm [range: 0-4.8 cm]. Among 11 evaluable patients, the cell of origin by Hans algorithm was GCB in 64%, non-GCB in 36% (Table). Staging procedures included brain and/or orbit imaging in 100% (MRI in 88%, CT in 12%), PET-CT in 94%, bone marrow evaluation in 69% (negative in all cases), and CSF evaluation in 47% (negative in all cases).
Sixteen patients were treated with R-CHOP and one with CHOP. Eleven patients received short-course chemotherapy (3 cycles in 7 patients, 4 cycles in 4), and 6 received full-course therapy (i.e., 6 cycles). Thirteen patients (76%) received radiation therapy (RT) to the ocular adnexa, including 9/11 patients treated with short-course treatment and 3/6 receiving 6 cycles. One patient with stage IIE disease received RT to the left neck after 3 cycles R-CHOP, but did not receive RT to ocular adnexum. Six patients received CNS prophylaxis, all with intrathecal methotrexate (Table).
First-line systemic therapy resulted in complete response (CR) in all patients. At a median follow-up of 64 months [range: 11-147], the 2- and 5-year PFS are 93% (95% CI 79-100%), and 84% (95% CI 64-100%), respectively, and 2- and 5-year OS are 100% and 91.7% (95% CI 76-100%), respectively (Figure). One of 17 patients had systemic relapse 18 months after first-line therapy and underwent salvage therapy followed by high-dose therapy and autologous stem cell transplantation, and remains in CR. There were no CNS relapses. Due to the paucity of events, we found no statistically significant associations between the number of chemotherapy cycles or the use of RT and PFS or OS. At the time of this analysis, four patients have died, none from lymphoma; one of the four died from acute myeloid leukemia 3 years after treatment.
Discussion
Our study demonstrates high response rates and highly favorable long-term outcomes in patients with limited stage OA-DLBCL treated with R-CHOP and RT. Our results are in line with those reported by Bobillo et al. in patients with stage 1 DLBCL involving other extranodal sites. With the limitation of a small sample size, short course R-CHOP was not associated with significantly worse response rates or increased risk of recurrence. Whether consolidation radiation therapy improves the results of chemotherapy alone remains to be determined in larger studies.
Batlevi: Dava Oncology: Honoraria; TG Therapeutics: Consultancy; Seattle Genetics: Consultancy; Epizyme: Research Funding; Novartis: Research Funding; Medscape: Honoraria; Viatris: Current holder of individual stocks in a privately-held company; Roche/Genentech: Research Funding; Regeneron: Current holder of individual stocks in a privately-held company; Pfizer: Current holder of individual stocks in a privately-held company; Memorial Sloan Kettering Cancer Center: Current Employment; Bayer: Research Funding; Kite Pharma: Consultancy; Karyopharm: Consultancy; ADC Therapeutics: Consultancy; TouchIME: Honoraria; Life Sciences: Consultancy; BMS: Current holder of individual stocks in a privately-held company; Janssen: Research Funding; Autolus: Research Funding; Moderna: Current holder of individual stocks in a privately-held company; Xynomic: Research Funding; GLG Pharma: Consultancy; Juno/Celgene: Consultancy. Caron: Astra-Zeneca: Current holder of individual stocks in a privately-held company; bristol myers: Current holder of individual stocks in a privately-held company; GlaxoSmithKlein: Current holder of individual stocks in a privately-held company; Johnson and Johnson: Current holder of individual stocks in a privately-held company; Novartis: Current holder of individual stocks in a privately-held company; pfizer: Current holder of individual stocks in a privately-held company; Teva: Current holder of individual stocks in a privately-held company. Hamlin: Kite, Karyopharm, Celgene: Membership on an entity's Board of Directors or advisory committees; Incyte, Janssen, Molecular Templates: Research Funding; Alexion, AstraZeneca Rare Disease (formerly Portola Pharmaceuticals): Other: Study investigator, Research Funding. Horwitz: Tubulis: Consultancy; Affimed: Research Funding; ONO Pharmaceuticals: Consultancy; Trillium Therapeutics: Consultancy, Research Funding; Acrotech Biopharma: Consultancy; Aileron: Research Funding; Shoreline Biosciences, Inc.: Consultancy; Celgene: Research Funding; Forty Seven, Inc.: Research Funding; Kyowa Hakko Kirin: Consultancy, Research Funding; SecuraBio: Consultancy, Research Funding; Verastem: Research Funding; C4 Therapeutics: Consultancy; Janssen: Consultancy; Kura Oncology: Consultancy; Millennium /Takeda: Consultancy, Research Funding; Myeloid Therapeutics: Consultancy; Daiichi Sankyo: Research Funding; Seattle Genetics: Consultancy, Research Funding; Vividion Therapeutics: Consultancy; ADC Therapeutics: Consultancy, Research Funding. Joffe: AstraZeneca. Epizyme: Consultancy. Khan: Seattle Genetics: Research Funding. Kumar: Celgene: Honoraria, Other: advisory board, Research Funding; Kite Pharmaceuticals: Other: advisory board , Research Funding; Astra Zeneca: Honoraria, Other: Advisory Board, Research Funding; Abbvie Pharmaceuticals: Research Funding; Seattle Genetics: Research Funding; Adaptive Biotechnologies, Celgene, Abbvie Pharmaceticals, Pharmacyclics, Seattle Genetics: Research Funding; Pharmacyclics: Research Funding. Matasar: Janssen: Honoraria, Research Funding; Teva: Consultancy; Juno Therapeutics: Consultancy; Merck: Consultancy; IGM Biosciences: Research Funding; Genentech, Inc.: Consultancy, Honoraria, Research Funding; Merck Sharp & Dohme: Current holder of individual stocks in a privately-held company; TG Therapeutics: Consultancy, Honoraria; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; Memorial Sloan Kettering Cancer Center: Current Employment; Rocket Medical: Consultancy, Research Funding; Pharmacyclics: Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy; Takeda: Consultancy, Honoraria; ImmunoVaccine Technologies: Consultancy, Honoraria, Research Funding; GlaxoSmithKline: Honoraria, Research Funding. Moskowitz: Miragen: Research Funding; Merck: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Janpix Ltd.: Consultancy; Imbrium Therapeutics L.P./Purdue: Consultancy; Bristol-Myers Squibb: Research Funding; Takeda: Consultancy; Incyte: Research Funding; Beigene: Research Funding; ADC Therapeutics: Research Funding. Noy: Medscape: Consultancy; Targeted Oncology: Consultancy; Morphosys: Consultancy; Rafael Parhma: Research Funding; Pharmacyclics: Consultancy, Research Funding; Janssen: Consultancy, Honoraria; Epizyme: Consultancy. Palomba: Wolters Kluwer: Patents & Royalties; BeiGene: Consultancy; Rheos: Honoraria; PCYC: Consultancy; Juno: Patents & Royalties; Kite: Consultancy; Pluto: Honoraria; Novartis: Consultancy; Notch: Honoraria, Other: Stock; Seres: Honoraria, Other: Stock, Patents & Royalties, Research Funding; Magenta: Honoraria; WindMIL: Honoraria; Lygenesis: Honoraria; Nektar: Honoraria; Ceramedix: Honoraria; Priothera: Honoraria. von Keudell: Merck: Consultancy, Honoraria; AbbVie: Research Funding; Pharmacyclics: Consultancy, Honoraria; BMS: Research Funding; Merck: Research Funding; Incyte: Consultancy, Honoraria; Janssen: Research Funding. Zelenetz: SecuraBio: Honoraria; Pharmacyclics: Honoraria; AstraZeneca: Honoraria; MEI Pharma: Honoraria, Research Funding; Verastem: Honoraria; NCCN: Other; Janssen: Honoraria; BMS/Celgene/JUNO: Honoraria, Other; Amgen: Honoraria; Gilead: Honoraria; Gilead: Honoraria, Research Funding; Genentech/Roche: Honoraria, Research Funding; MethylGene: Research Funding; MorphoSys: Honoraria; Abbvie: Honoraria, Research Funding; LFR: Other; Beigene: Honoraria, Other, Research Funding; Novartis: Honoraria. Dogan: Roche: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Peer View: Honoraria; EUSA Pharma: Consultancy; Seattle Genetics: Consultancy; Physicians' Education Resource: Honoraria. Salles: Debiopharm: Consultancy; Takeda: Consultancy; Allogene: Consultancy; Velosbio: Consultancy; Genmab: Consultancy; Loxo: Consultancy; Miltneiy: Consultancy; Ipsen: Consultancy; Janssen: Consultancy; Kite/Gilead: Consultancy; Incyte: Consultancy; Morphosys: Consultancy, Honoraria; Novartis: Consultancy; Rapt: Consultancy; Regeneron: Consultancy, Honoraria; Genentech/Roche: Consultancy; Epizyme: Consultancy, Honoraria; BMS/Celgene: Consultancy; Beigene: Consultancy; Abbvie: Consultancy, Honoraria; Bayer: Honoraria.